
Veeda Group has a custom cell line, positive controls, and other reagents that are necessary for the development of robust Nab assays. We utilize cell-based methods based on the drug’s mechanism of action, such as cell proliferation, cell viability, cell signalling, receptor activation, etc., to develop and validate Nab assays. In addition to cell-based methods, we also have developed innovative ligand binding methods. For the evaluation of neutralizing antibodies, that has been accepted by global regulatory authorities.
Veeda Group offers high-quality, sensitive, and robust off-the-shelf methods and ELISA kits for the measurement of biomarkers in biological matrices. Our assays are designed to minimize the effect of different biological matrices on the performance of the assay while maintaining appropriate levels of sensitivity.


We are a quality-driven, responsive partner with deep scientific and regulatory expertise in the area of large molecule bioanalysis, including vaccines. We combine our extensive therapeutic and laboratory expertise with our resources to provide vaccine development services of a high caliber to our global clientele.
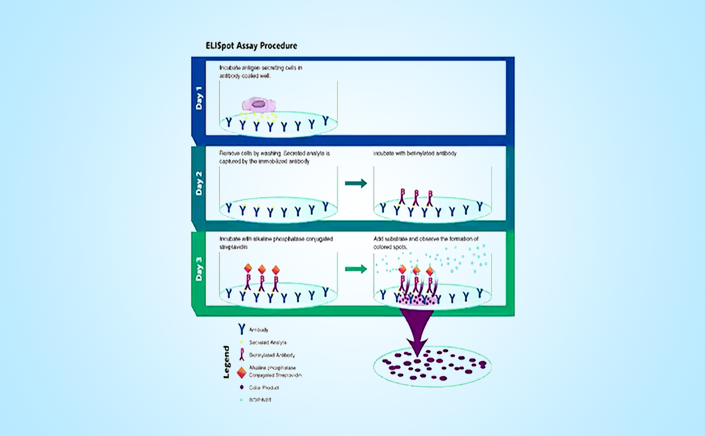
Cell-mediated immunity for vaccine-specific immune response monitoring.
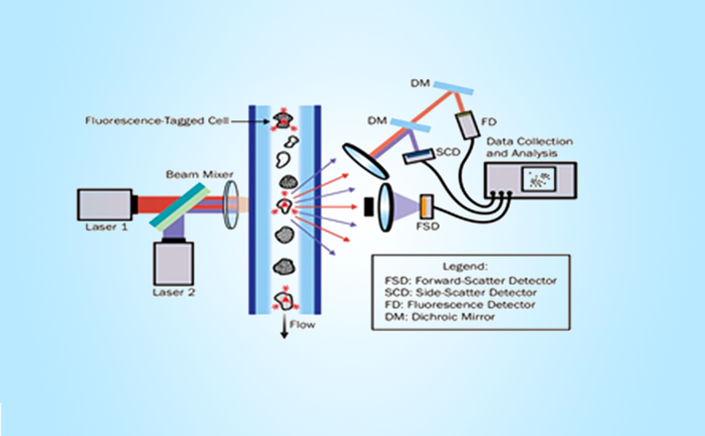
Flow cytometry is a technique that can provide quantitative and qualitative information about biological cells and micro particles. Population functional analysis of immune response post administration.
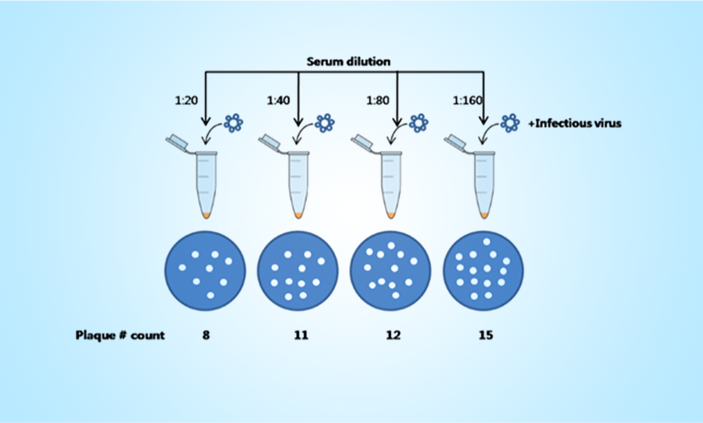
Measures neutralizing antibodies by in vitro virus neutralization.
PRNT has been considered the gold standard for assessing viral neutralization for many viral diseases utilizing wild-type virus in highly controlled biocontainment laboratories. This method is used widely to measure the neutralization activity of antibodies of large molecules (Vaccines).
PNA (updated version of PRNT) Assay is, dedicated to provide biopharmaceutical services for characterization and bioanalytical testing in support of preclinical and clinical development of vaccines and other novel biologics drug products.
| PRNT Limitations | PNA Advantages |
|---|---|
| High level of biosafety containment (Level 3 minimum) not accessible to most bioanalysis facilities | Performed with lower biocontainment requirement (Level 2 minimum) accessible to most bioanalysis facilities |
| Technically demanding | Utilization of common cell culture and immunoassay skills |
| Has very low throughput and difficult to automate | Increased throughput due to 96 well plate format which can be automated |
| Long turnaround time due to the time taken for the virus to form visible plaques | Shortened turnaround time: Reducing assay time to ~24 hour rate than up to 5 days |
| The analysis of the count data is typically performed using Excel leading to difficulties in using the data as part of a regulatory submission as data should be analyzed by validated software | Typical regression curve analysis common to all bioanalysis labs and software |
