Clinical Bioanalysis of NBEs & Biosimilars
Veeda Biopharma is your trusted partner for comprehensive Clinical Bioanalysis services for Novel Biotherapeutic Entities (NBE) and Biosimilars for emerging biopharma companies globally, with a focus on key regions such as the USA, Europe & India. Our advanced capabilities encompass a range of critical assessments, including Clinical Biomarkers, In-vitro Pharmacodynamics (PD), Pharmacokinetics (PK), Anti-Drug Antibody (ADA), Cytokine Response, Neutralizing Antibody Assays (NAb) utilizing science driven Bioanalytical Method Development followed by Method Validation as per ICH M10 requirements. Veeda Biopharma provides an accurate and precise result for all solutions under a single roof, contributing significantly to a thorough understanding of innovative medicinal products with safety and efficacy profiles.

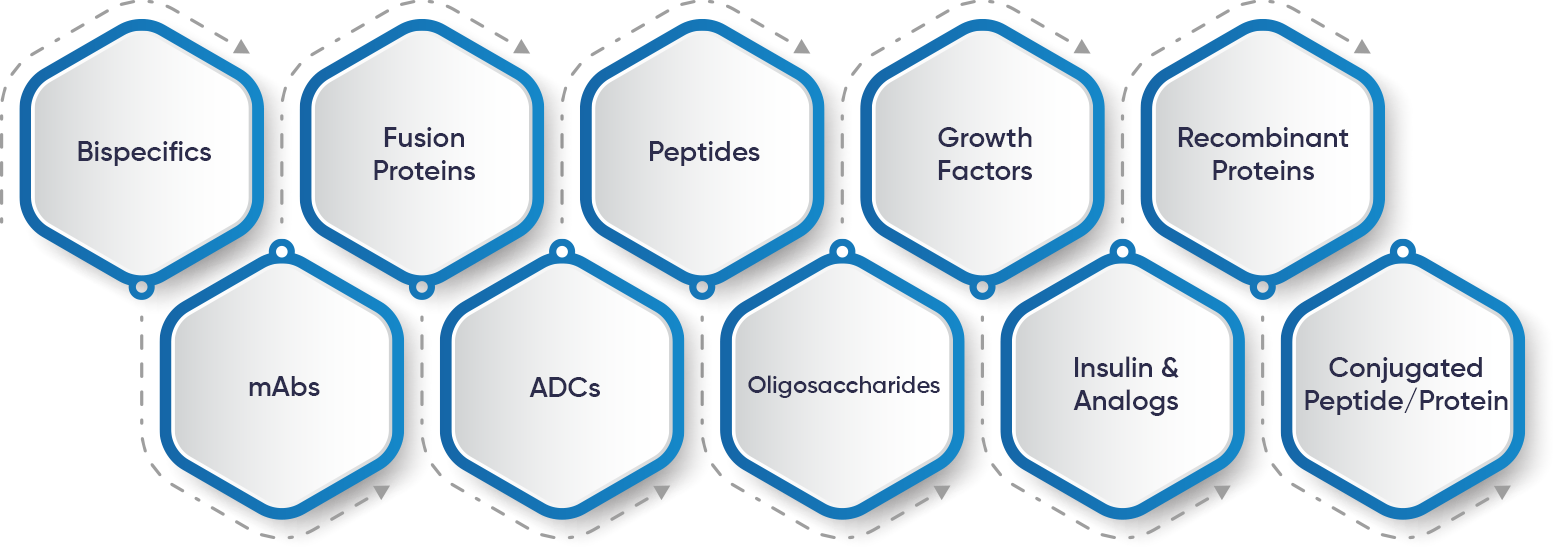
Our Infrastructure for Customized Integrated Solutions: Tailored for your Biopharma R&D Success

Pharmacokinetic and
Pharmacodynamic Analysis
PK and PD analysis study relates drug exposure to therapeutic outcomes to help drug developers to understand the relationships between exposure, efficacy, and toxicity of a particular drug. Thus, PK/PD data analysis results are highly crucial and essential for any eCTD submission.
Pharmacokinetic/Pharmacodynamic assessments involve estimation of biotherapeutics in human serum/plasma. Specialized in evaluating PK/PD parameters using compliant statistical software for their primary and secondary endpoints like Cmax and AUCO-inf, AUCO-t, tmax, t1/2, Vd and CL etc as per the sponsor’s requirements.
Anti-drug Antibody Assays: Method Transfer
& Development for Biologics and Biosimilars


Bridging Anti-Drug Antibody
Assay Development
Assay Development

Using Meso Scale Discovery or ELISA for MAbs, Peptide, ScFv, or Protein, with in-house tagging (Ruthenylation, Biotinylation) of critical reagents.
Using Meso Scale Discovery or ELISA for MAbs, Peptide, ScFv, or Protein, with in-house tagging (Ruthenylation, Biotinylation) of critical reagents.
Improving the estimation of Drug Tolerance by Acid Dissociation, Solid-Phase Extraction with Acid Dissociation (SPEAD), Affinity Capture Elution (ACE), Biotin-Drug Extraction and Acid Dissociation (BEAD), Precipitation and Acid Dissociation (PandA).
Improving the estimation of Drug Tolerance by Acid Dissociation, Solid-Phase Extraction with Acid Dissociation (SPEAD), Affinity Capture Elution (ACE), Biotin-Drug Extraction and Acid Dissociation (BEAD), Precipitation and Acid Dissociation (PandA).
High-Quality ADA Assay Validation
Validation based on a Multi-Tiered Approach, i.e., Screening, Confirmation, and Titration Cut Point as per FDA's Guidance on Immunogenicity Testing of Therapeutic Protein Products.
Validation based on a Multi-Tiered Approach, i.e., Screening, Confirmation, and Titration Cut Point as per FDA’s Guidance on Immunogenicity Testing of Therapeutic Protein Products.

Risk Based Immunogenicity Assessment for Clinical Samples
- ADA screening assay to detect high and low-affinity anti-drug antibodies
- ADA Titer assay to determine the magnitude of ADA response
- ADA Confirmatory assay to eliminate false positives from nonspecific binding
- ADA Neutralizing Antibody (NAb) assay to assess the neutralizing activity
Neutralizing Antibody Assay Development




Biomarker Analysis: Multiple Platform Support for
Cytokine & Biomarker Studies


Cytokine/Biomarker Assay Development, Validation and Sample Analysis
Assay Development using ELISA, Meso-Scale Discovery, Luminex, Flow Cytometry & commercially available kits or custom designed with in-house conjugation services
Method Validation following ICH Guidelines using qualified equipment and reagents