Development and Execution of In Vivo Bioassays
January 8, 2022
Home > Veeda Insights > Development and Execution of In Vivo Bioassays

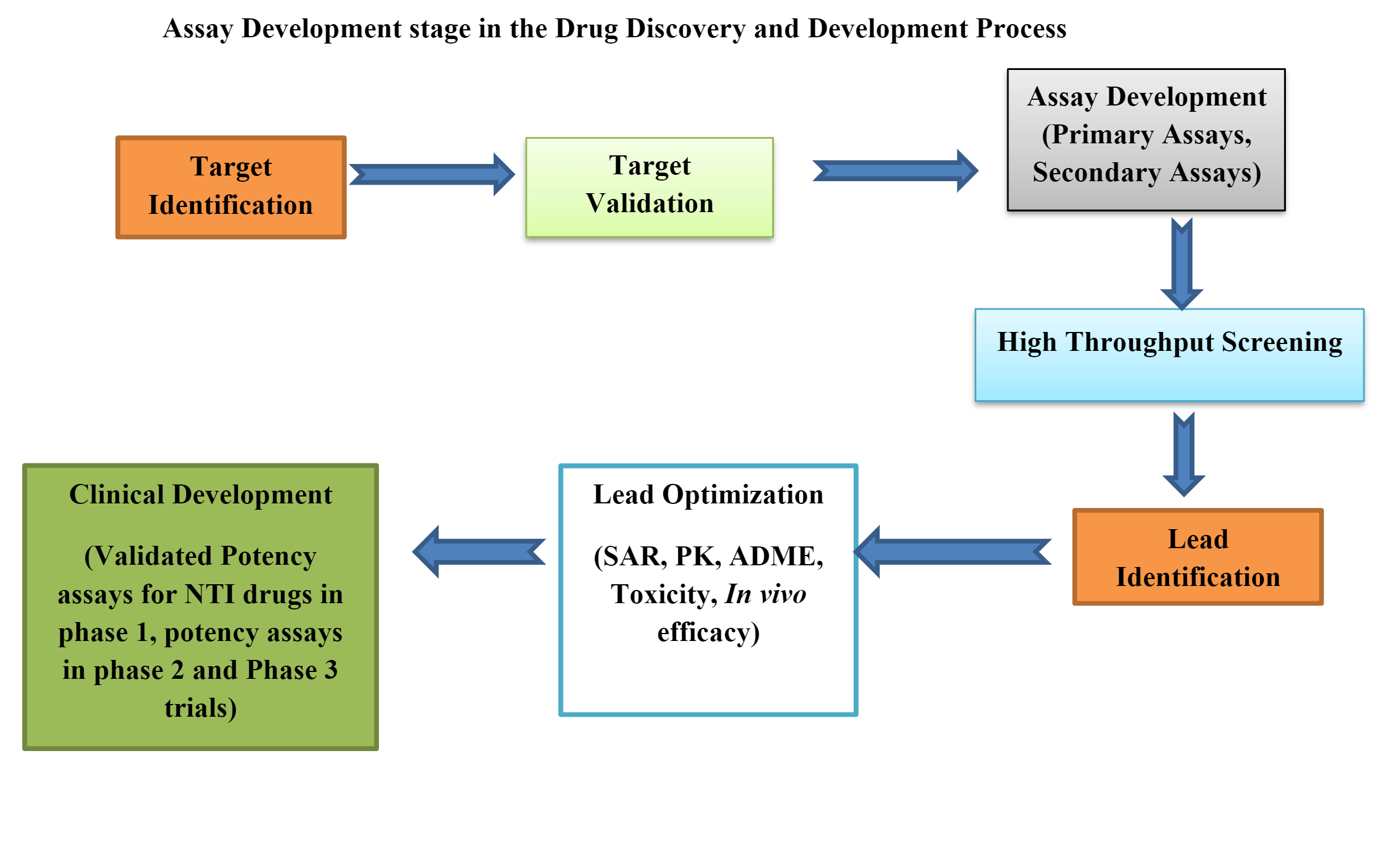
Bioassays are involved in each stage of drug discovery, starting from Target Identification until discovering the Lead compound. Bioassays provide valuable information which displays the therapeutic potency of a drug under investigation. The data generated during bioassay also plays a vital role in drug development and quality control of finished biological products. Properly designed Bioassays help in assessing the biological effect, activity, signal transduction process and receptor binding ability of drug product or biologic on a biological target (proteins) when compared to a reference or standard over a suitable biological system.
The pharmaceutical and biotech companies involved in drug discovery and development are continuously challenged with developing biologically relevant assays for the analysis of multiple potential mechanisms. The process involves use of quality critical reagents, use of specific cell lines and purified test drug and reference drug products which at times may become a constraint. Most of these activities require sufficient time which may become a limiting factor to biopharma manufacturers. It is worth outsourcing activities to reputed CRO service providers to save time in developmental efforts and also to have an unbiased opinion on the functional activities of the drug product.
Veeda Group has qualified and experienced scientists to design, develop, execute, and validate the Bioassays for companies and provides premier bioassay services (in vitro and in vivo) that generate meaningful data to support pharmaceutical and biotech companies in their drug discovery and development journey.
Veeda Group’s experience in Development and Execution of Bioassays include:
Veeda Group provides Integrated Discovery, Development & Regulatory Services with its multiple technology platforms:
The group also has the experience to handle diverse range of Biotherapeutics like Therapeutic Monoclonal Antibodies, Insulin & Insulin Analogues, Cytokines, Low Molecular Weight Heparins, Biosimilars, Hormones & Biomarkers. Veeda group has demonstrated capabilities to develop recombinant proteins such as non-glycosylated proteins and glycoproteins derived from either bacterial or mammalian host expression systems.
Bioassays in Preclinical Drug Development
Biological assays or bioassays are an essential tool in preclinical drug development. Preclinical bioassays can be in vivo, ex vivo, and in vitro. In vivo bioassays provide a more realistic and predictive measure of functional effects of tests with reference drug products or standard material of defined potency along with the application of statistical tools, study-specific lab techniques, and adherence to the well-designed study protocol. These assays capture the complexity of target engagement, metabolism, and pharmacokinetics of novel drugs better than in vitro bioassays.
The most commonly used experimental mammals in in vivo efficacy assays are mice and rats. Occasionally other species may be used depending on the sensitivity & suitability of the assays.
Development and Validation of Bioassays
Bioassays are used as a screening method to identify the signals that indicate desired biological activity from a set of compounds. In general, two different types of signals can be generated by a bioassay, a linear dose-response, and a sigmoidal (S-shaped) dose-response. Since one solution does not fit all bioassays, it is good to evaluate and analyze the data to develop a precise approach to carry out each bioassay. The life cycle stages of a bioassay are divided into:
Stage 1: Method design, development, and optimization
Stage 2: Procedure performance qualification
Stage 3: Procedure performance verification (fit for purpose)
Developing a bioassay that meets regulatory requirements and gets drug product registered is a very complex process. Developing a bioassay, includes many strategies and tactical designs like selecting the correct in vivo platform, proper method or plate design, data analysis, system/ sample sustainability strategy, method implementation, method performance and monitoring. There are several steps to be followed for development and validation of bioassays such as dose response and curve-fitting selection, development of reference, calculation of potency, bioassay characterization, design of bioassay calculator, standardization and automation of bioassay and finally evaluation.
Both method development and validation of bioassays include three fundamental areas:
During method development, assay conditions and procedures are selected that minimize the impact of potential sources of invalidity. Coming to the statistical validation for an in vivo assay, it involves four major components:
Parallel group design, randomized block design, repeated measures design, and crossover design are the basic types of experimental designs used in in vivo assay. Following are the key-factors that should be kept in mind while designing an in vivo assay
For a 4PL model, nine doses are recommended:
In contrast, for a PLA model, a minimum of four doses is recommended. A minimum of three consecutive doses is required to plot the dose curve.
To understand design, developments, and statistical validation of in vivo bioassay in more details, reach out to us at https://www.veedacr.com/uk/ . One can also read the guidelines mentioned by NIH by visiting the link:
https://www.ncbi.nlm.nih.gov/books/NBK92013/pdf/Bookshelf_NBK92013.pdf
References