
- Knowledge-based, quality oriented staff with combined Team Experience in Clinical Trials of more than 130 clinical trials that includes around 25 global clinical trials, around 30 clinical endpoint studies and 75 patient based PK clinical trials.
- Database of more than 600 pre-screened experienced and GCP-trained Investigators / sites in various therapeutic areas.
- Study Design and Protocol Development Services.
- In-depth feasibility evaluation with potential Investigators' sites
- Project managers and CRAs experienced in planning, executing Clinical endpoint studies, Pharmacokinetic studies in patient population and Phase II / III studies.
- Key opinion leaders (KOLs) onboard - Consultants with vast experience in study design, conduct and safety aspects of Clinical Trials
With experienced team at COD, experienced Investigators and robust QA systems, we have successfully faced total fifteen (15) USFDA inspections at Investigator sites (at which Veeda had conducted PK studies) with only one minor 483 observation, for which no action was required. |
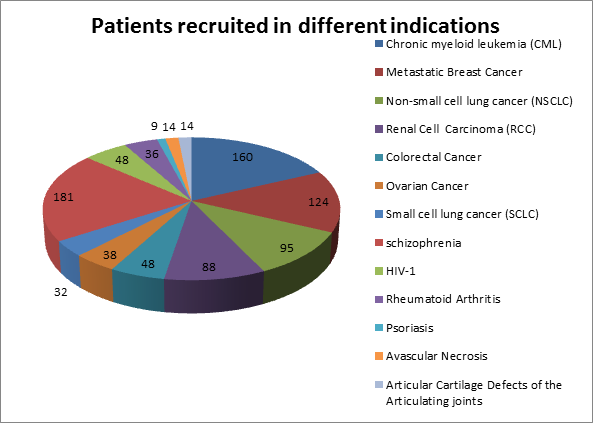 |
Some of the therapeutic segments where we can initiate studies at a shorter notice are
|
|
Veeda's Investigators Database has access to more than 600 potential Investigators' site across India in below therapeutic areas. | 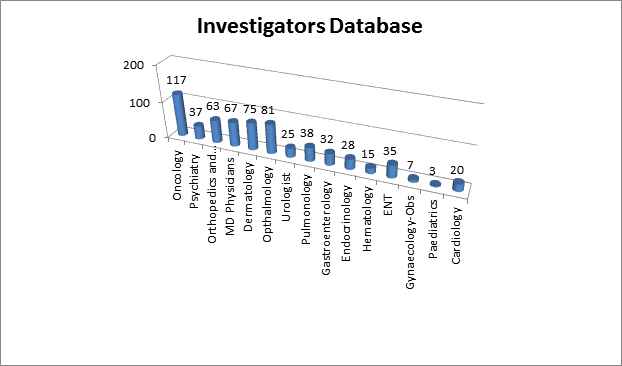 |
Our Achievements :
|
Vedant Complex, Beside YMCA club S.G.Highway, Vejalpur,Ahmedabad-380051,Gujarat India
 +91 79 3001 3000 |
+91 79 3001 3000 | .png) +91 79 3001 3010 |
+91 79 3001 3010 | .png) info@veedacr.com |
info@veedacr.com |
 www.veedacr.com
www.veedacr.com
